Reevaluation of optimal dietary arginine levels to support maximum broiler performance and arginine role on wound healing
2020.06.10

INTRODUCTION
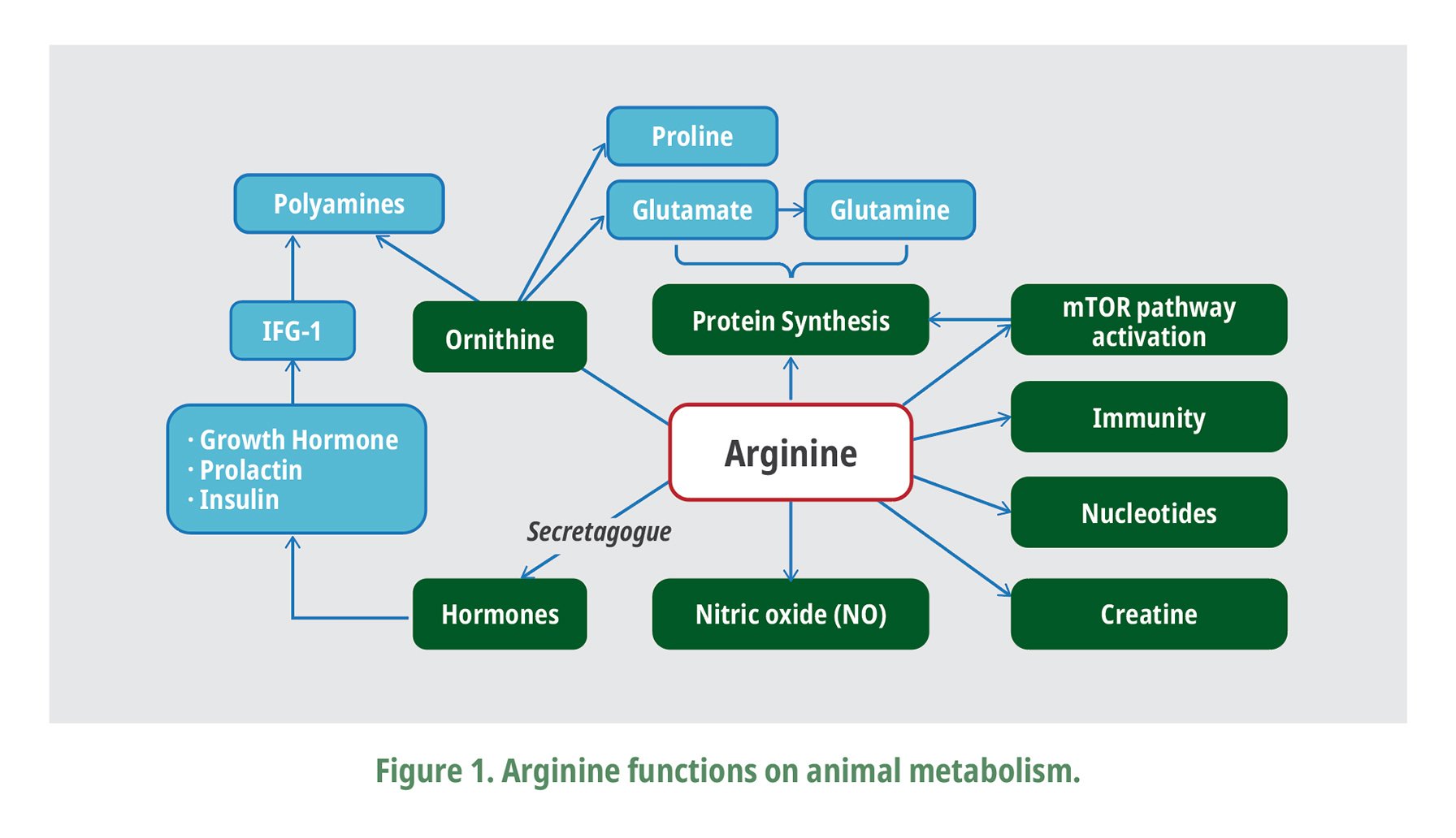
Dietary arginine supplementation higher than the recommended levels was shown to improve broiler performance (Murakami et al. 2012; Xu et al., 2018; Zampiga et al., 2018). It suggests that arginine levels needed for maximum performance could be higher in modern broilers, which is probably related to the several functions of arginine on animal metabolism (Fig. 1). Besides, arginine enhances the protein synthesis via the activation of the mTOR signaling pathway (Yao et al., 2008; Yuan at., 2015); this amino acid is a precursor for critical molecules such as nitric oxide, creatine (Chamruspollert et al., 2002), polyamines, proline, glutamate, citrulline, and agmatine (Wu and Morris, 1998). Arginine regulates key aspects of animal growth by enhancing insulin, growth hormone, and insulin-like growth release (Cochard et al., 1998; Xu et al., 2018). In addition, arginine supplementation has shown to increase breast meat yield (Corzo et al., 2003; Jiao et al., 2010) while reducing wooden breast myopathy incidence in modern fast-growing broilers (Bodle et al., 2018; Zampiga et al., 2018).
Optimal dietary arginine levels for broiler performance
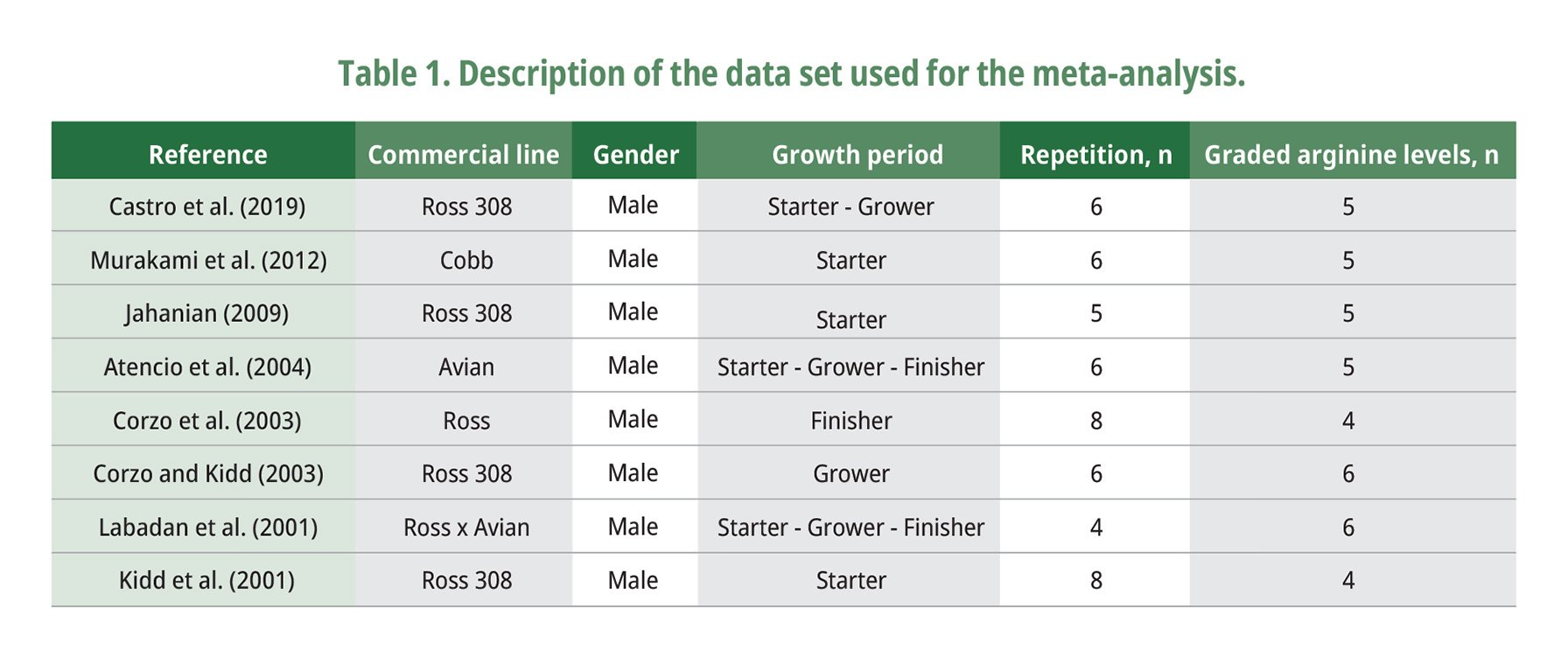
A meta-analysis based on 94 observations from eight peer-reviewed papers was conducted to determine the optimal arginine levels in broiler diets. The arginine base data included peer-reviewed papers between 2001 and 2019 (Table 1). When not given the standardized ileal digestible (SID) amino acid contents of the experimental, they were calculated using digestibility coefficients of ingredients as proposed by Rostagno et al. (2017).
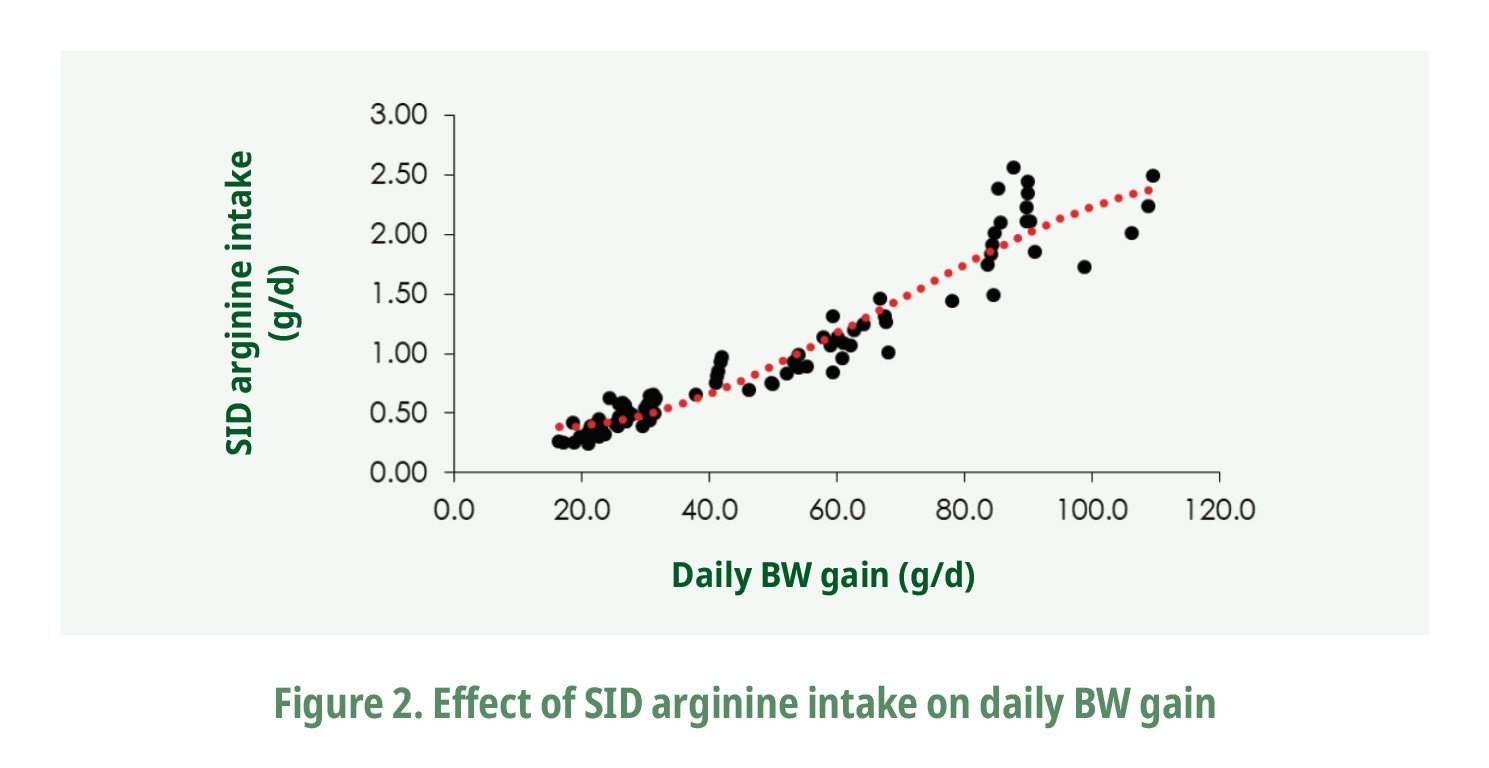
BW gain data (g/d) plotted against arginine intake (g/d) showed a positive response in BW gain as increasing arginine intake (Fig. 2). A significant quadratic regression (P=0.0138) was fitted from the 94 observations to predict the daily SID arginine intake to achieve a desirable BW gain during the different growth phases. For instance, an optimal level of SID arginine of 1.45% would be needed to achieve a daily BW gain of 19.8 g in broilers from 1 to 7 days of age (Table 2).

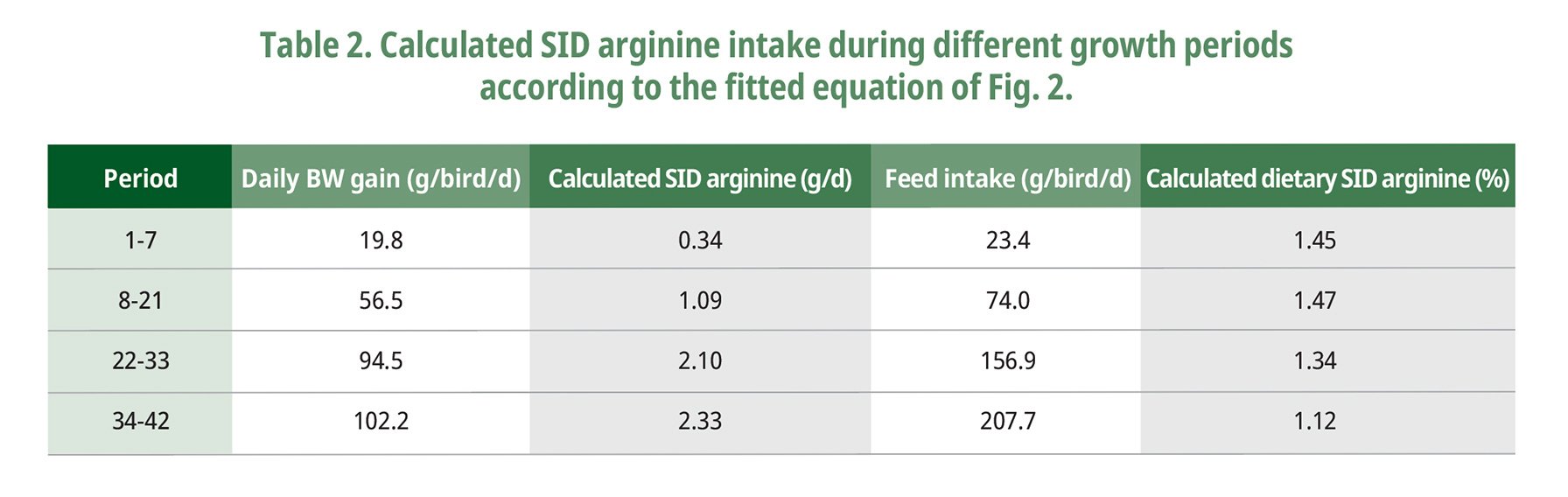
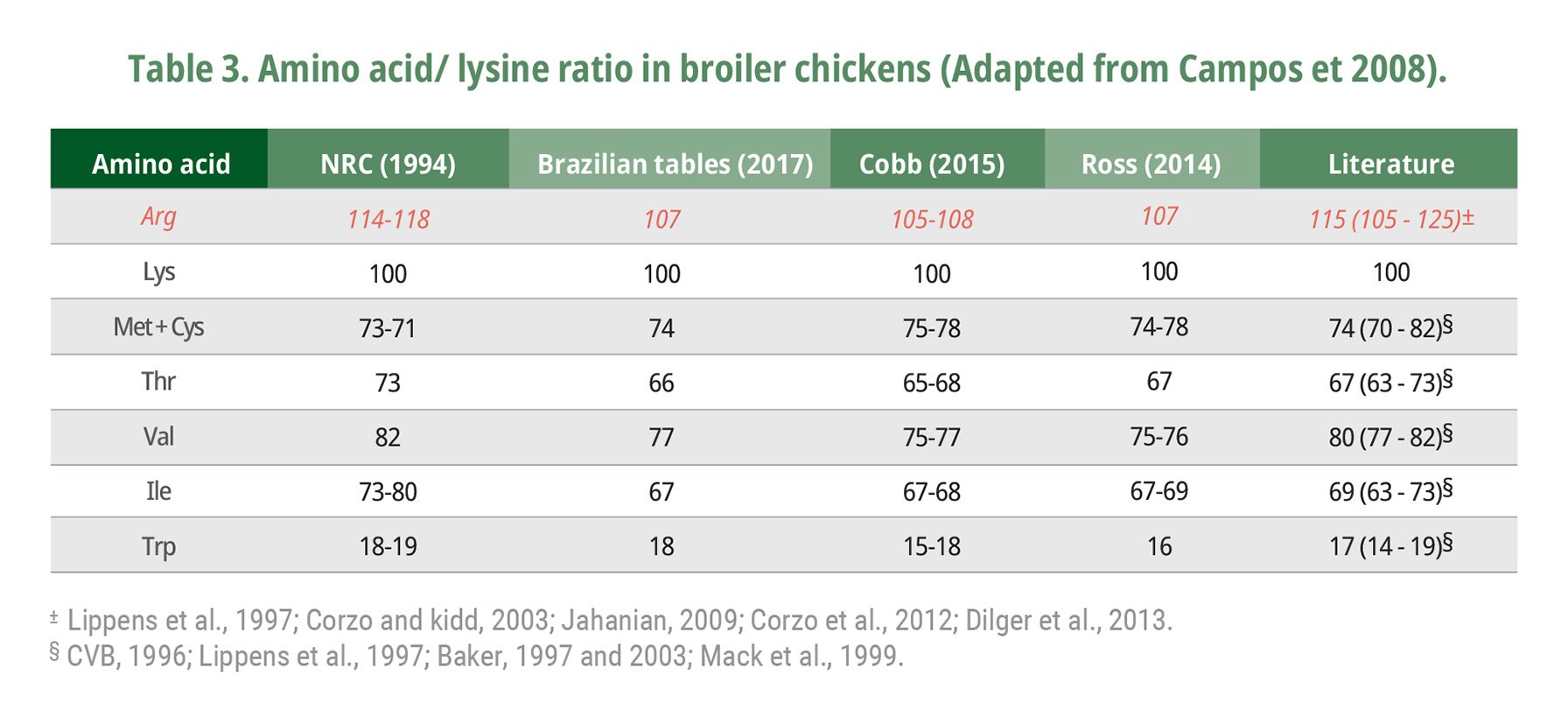
Considering a SID lysine recommendation of 1.28% and 1.07% for birds from 1 to 21 days and 22 to 42 days according to Rostagno et al. (2017), the ideal SID arginine:lysine ratio could be estimated at 114 and 115 for broilers during the starter and grower periods, respectively. Recommendations of arginine:lysine ratio range in the literature from 105 to 125, depending on the variable studied, with a mean of 115% (Table 3) (NRC, 1994, Lippens et al., 1997; Baker, 1997; Kidd et al., 2001; Corzo and Kidd, 2003; Jahanian, 2009; Corzo et al., 2012). The SID arginine:lysine ratio to optimize the immune system response is higher than that for optimal feed conversion which is higher than for maximum BW gain (Corzo et., 2003; Jahanian, 2009). Corzo et al. (2012) indicated that broiler chicks optimize their BW gain and feed conversion values at arginine:lysine ratios of 108 and 114, respectively. The SID arginine:lysine ratio estimated from the nutritional recommendations of Jahanian (2009) was 115, 117, and 122 to optimize BW gain, feed conversion, and immune system function, respectively.
Arginine supplementation as a strategy to reduce broiler carcass condemnation
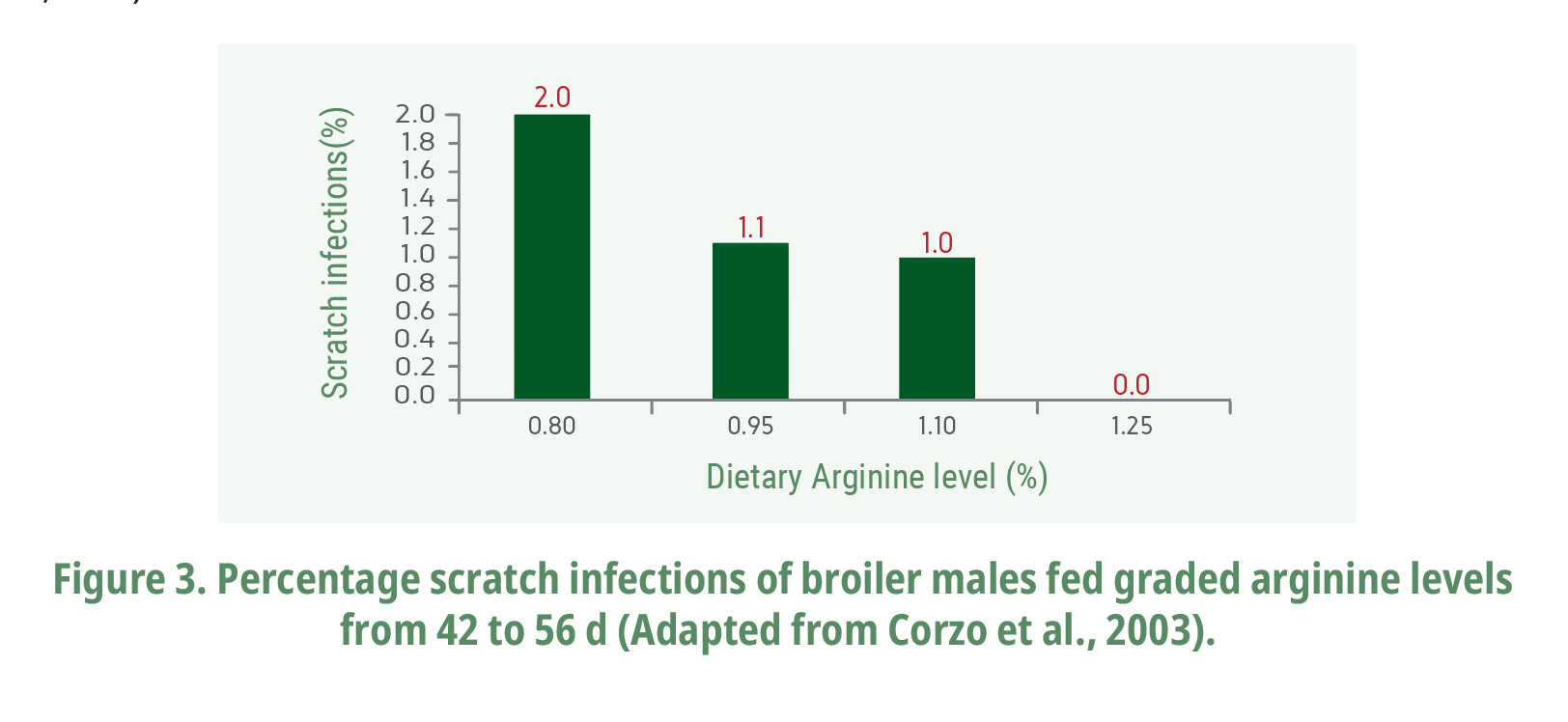
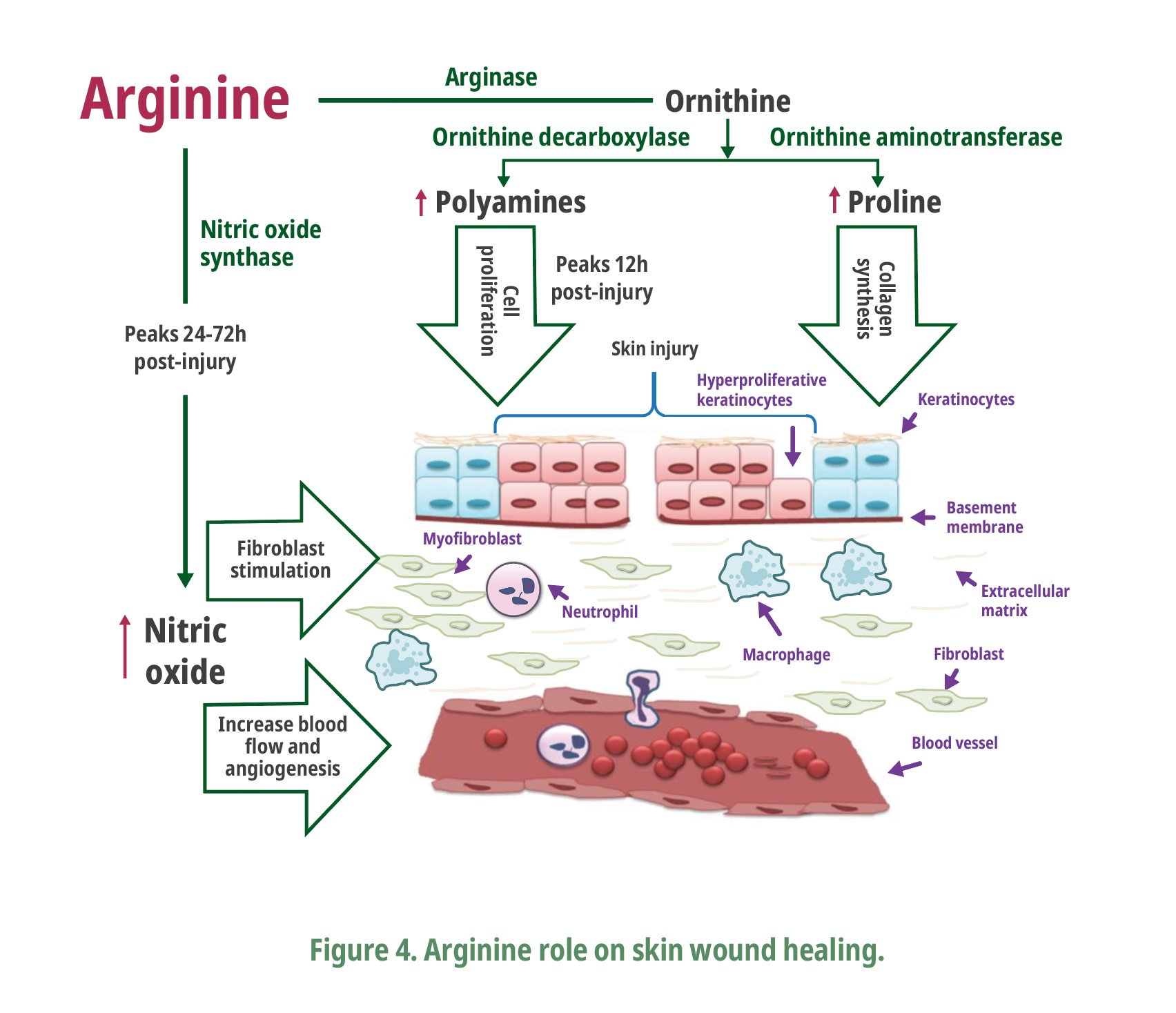
Interestingly, Corzo et al. (2003) found a linear reduction in infected carcass scratches of broilers fed arginine-supplemented diets (Fig. 3). Infected skin scratches as cellulitis are considered the first major reason for condemnation at slaughter followed by fractures/contusion (Santana et al., 2008), which is associated with significant economic losses in poultry industry. The reduced infected scratches in arginine-supplemented diets are not only related to the good immunity status as well as to a dermis with greater resistance. The arginine role in enhanced wound healing has been well-documented in mammals by increasing breaking strength and reparative collagen accumulation (Shi et al, 2003; Tong et al., 2004; Stechmiller et al., 2005) (Fig. 4). For instance, the production of nitric oxide synthase peaks within 2472 h after wounding (Stechmiller et al., 2005), producing nitric oxide, and citrulline, where nitric oxide plays a major role in antimicrobial activity and improving blood flow to the healing wound (Alexander and Supp, 2014). Thereafter, arginase activity increases with a marked rise in wound ornithine levels which is metabolized to polyamines by ornithine decarboxylase (Shi et al., 2002). The polyamine concentrations increase within 12 h after skin incisional wounding in rats and they are factors necessary for cellular growth involved in tissue repair and wound healing (Maeno et al., 1990). The inhibition of ornithine decarboxylase, an enzyme related to polyamine synthesis, impairs skin wound healing (Lesiewicz and Goldsmith, 1980).
In addition, arginine is a metabolic precursor of proline and hydroxyproline, the major amino acids in the collagen proteins, which are major extracellular components in connective tissues of skin and tendons (Wu et al., 2011). Fernandes (2007) reported a linear increase in broiler dermis thickness as increased dietary arginine levels. It suggests the importance of arginine in skin breaking strength. Scratches with no dermis penetration resulted in a more resistant dermis having less chance to be infected. The effect of arginine supplementation should be further studied considering the huge economic benefits that it may bring to poultry production to reduce carcass condemnation.
CONCLUSION
Arginine has a vast variety of effects on metabolism, including protein synthesis, immunity, wound healing, hormone secretion, neurotransmission, normal cell differentiation, and growth, vasodilation, and antioxidant activity. Several of these effects are mediated by nitric oxide that elicits a surprisingly wide range of physiological functions and arginine is the only effective precursor in birds. Broilers are usually exposed to various stressors during their rearing period (vaccination, cold or heat stress, immune challenge, and high stocking density among others) that results in economic expenses by decreased growth and increased susceptibility to diseases. Because dietary arginine levels could be insufficient for a broilers metabolic needs during stress periods, arginine supplementation has been considered important to attenuate reduced performance of broilers in these conditions. Dietary arginine:lysine ratio was estimated at 114 and 115 for optimal performance of broilers during the starter and grower phases, respectively. However, these ratios may be greater to maximize immune system function and skin breaking strength. Higher arginine levels may be an interesting strategy to minimize carcass condemnation issue in the poultry industry, reducing the infected scratches and improving wound healing as well as carcass quality.
REFERENCES
1. Alexander JW, and Supp DM. 2014. Ad Wound Care. 3:682690.
2. Atencio A, et al. 2004. R Bras Zootec. 33:1456-1466.
3. Baker DH. 1997. BioKyowa Tech Rev. 9:124.
4. Baker DH. 2003. DMello JPF, ed. CABI Publishers, Wallingford, UK.
5. Bodle BC, et al. 2018. Poult Sci. 97: 3298-3310.
6. Castro FLS, et al. 2019. Poult Sci. 98:1716-1722.
7. Chamruspollert M, et al. 2002. Br J Nutr. 88:655-660.
8. Cochard A, et al. 1998. Reprod Nutr Dev. 38: 331343.
9. Corzo A, and Kidd MT. 2003. Int J Poult Sci. 2:379-382.
10. Corzo A, et al. 2003. Poult Sci. 82:402407.
11. Corzo A. 2012. J Appl Poult Res. 21:79-87.
12. CVB (Centraal Veevoeder Bureau), Netherlands. 2000.
13. Dilger RN, et al. 2013. Poult Sci. 92:171-177.
14. Fernandes JIM. 2007. PhD Diss., Universidade Estadual de Maring, Maring.
15. Jahanian R. 2009. Poult Sci. 88:1818-1824.
16. Jiao P, et al. 2010. J Anim Vet Adv. 9: 1546-1551.
17. Kidd MT, et al. 2001. Poult Sci. 80:1535-1542.
18. Labadan MC Jr, et al. 2001. Poult Sci. 80: 599-606.
19. Lesiewicz J, and Goldsmith LA. 1980. Arch Dermatol. 116: 1225.
20. Lippens M, et al. 1997. Agricultural Research Department.
21. Mack S, et al. 1999. Br Poult Sci. 40:257265.
22. Maeno Y, et al. 1990. Forensic Sci Int. 46: 255.
23. Murakami AE, et al. 2012. Pesq Vet Bras. 32: 259-266.
24. Rostagno HS, et al. 2017. 4ª ed. UFV, Viosa, MG, Brazil. 488 p.
25. Santana AP, et al. 2008. Cienc Rural 38: 2587 2592.
26. Shi HP, et al. 2002. J Surg Res. 106:299-302.
27. Shi HP, et al. 2003. Wound Repair Regen. 11:198203.
28. Stechmiller JK, et al. 2005. Nutr Clin Pract. 20:52-61.
29. Tong BC, and Barbul A. 2004. Mini Rev Med Chem. 4:82332.
30. Wu G, and Morris SM Jr. 1998. Biochem J. 336:117.
31. Wu G, et al. 2011. Amino Acids. 40: 10531063.
32. Xu YQ, et al. 2018. Livest Sci. 209, 813.
33. Yao K, et al. 2008. J Nutr. 138:867872.
34. Yuan C, et al. 2015. Poult Sci. 94:1043-51.
35. Zampiga M, et al. 2018. J Anim Sci Biotechnol. 9:79.























