Metabolic and performance benefits of L-Arginine over Guanidinoacetic acid
2020.03.17

Introduction
There are at least five commercially available synthetic amino acids, namely methionine, lysine, threonine, tryptophan and valine. Currently, arginine (Arg) is available which is produced through bio-fermentation. L-Arg is an essential amino acid for chickens due to unavailability of two separate enzymes in urea cycle in the kidney and almost all urea cycle enzymes in the liver (Leeson and Summers, 2001). The mammalian cells have a fully functional urea cycle. Therefore, L-Arg is not considered as an essential amino acid in swine. However, new data shows that endogenous arginine synthesis through urea cycle is not enough to cover arginine requirements in swine (Wu et al., 2007). Thus, arginine is named to be essential e.g. in piglets as well.
Guanidinoacetic acid (GAA) is the precursor of creatine which is synthesized via a two steps process involving two enzymes and three amino acids: arginine, glycine, and methionine. In the first step, arginine : glycine amidinotransferase (AGAT) transfers an amidino group from arginine to the amino group of glycine to produce guanidinoacetate (GAA) and ornithine:

Whereas, in the second step, GAA methyltransferase (GAMT) employs S-adenosylmethionine (SAM) to methylate GAA, producing creatine
and S-adenosylhomocysteine (SAH):

L-Arg and GAA are used in practice in order to cover arginine requirements of the animals. Thus, understanding the differences between
the two products is important.
Metabolism of L-Arg and GAA
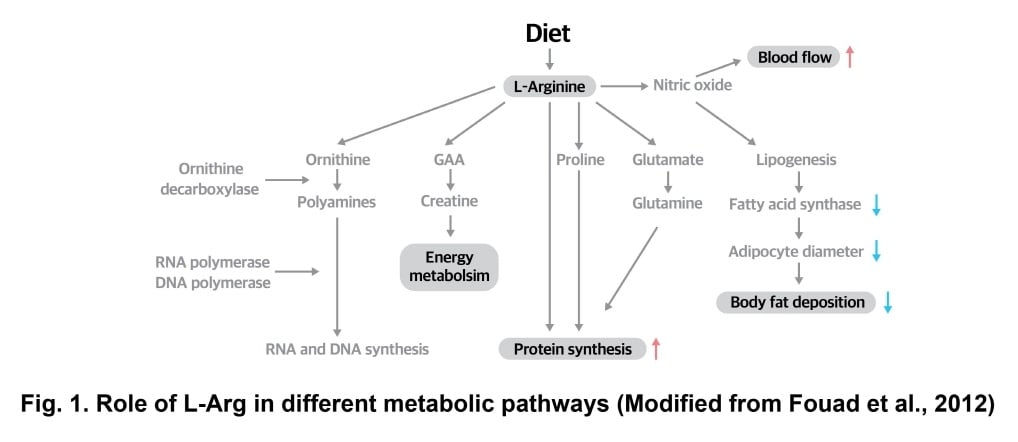
L-Arg not only serves as building blocks of proteins e.g. in muscles but also performs several other vital metabolic functions. Moreover, L-Arg serves as a substrate for the biosynthesis of different molecules such as nitric oxide, GAA, glutamate, ornithine and proline. Nitric oxide as an important mediator of vasodilation contributes to an increase of blood flow to the organs. Nitric oxide also reduces fat synthesis and promotes fat oxidation (Jobgen et al., 2006). L-Arg also improves the carcass yield especially the breast muscles in broilers via formation of glutamate and proline (Khajali and Wideman, 2010). Glutamate, proline and hydroxyproline are also required for the synthesis of connective tissues (Khajali and Wideman, 2010). Similarly, other molecules synthesized from the L-Arg metabolism including ornithine and polyamines contribute in DNA and RNA synthesis for normal cellular growth (Chen et al., 2011).
GAA is an endogenous metabolite of Arg which participate in muscle energy buffering system (Khajali and Wideman, 2010; Chen et al., 2011)
(Fig. 1). High cellular concentration of GAA has a negative feedback on AGAT, thus endogenous synthesis of GAA will not happen when
enough GAA is added through the diet (Takeda et al., 1992).
Comparative performance studies
Study I:
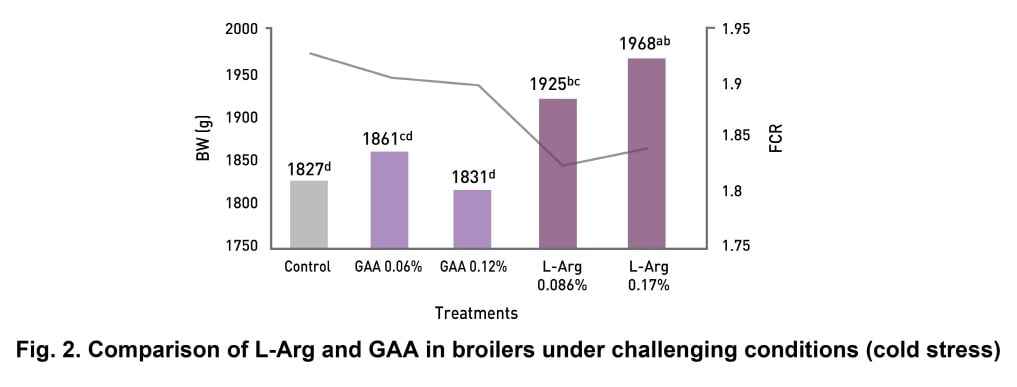
Ascites, cardiovascular metabolic disorder, is a major challenge under cold stress conditions. It was hypothesised that providing extra Arg can reduce ascites incidences in broilers (Saki et al., 2013). Emami et al. (2016) investigated the effect of L-Arg (0.86-1.72 g/kg of feed) and GAA (0.6-1.2 g/kg of feed) under cold stress condition. The digestible Arg to digestible Lys ratio was 107:100 in the basal diet. The temperature of one house was according to optimal conditions, whereas, the temperature in the second house was gradually decreased to 17 °C from d 14 onward to the end of experiment in order to induce ascites. L-Arg group significantly (p<0.05) increased body weight (BW) and reduced feed conversion ratio (FCR) as compared to the basal diet and the GAA group (Fig. 2). The authors
concluded that arginine can help in broiler production under challenging conditions.
Study II:
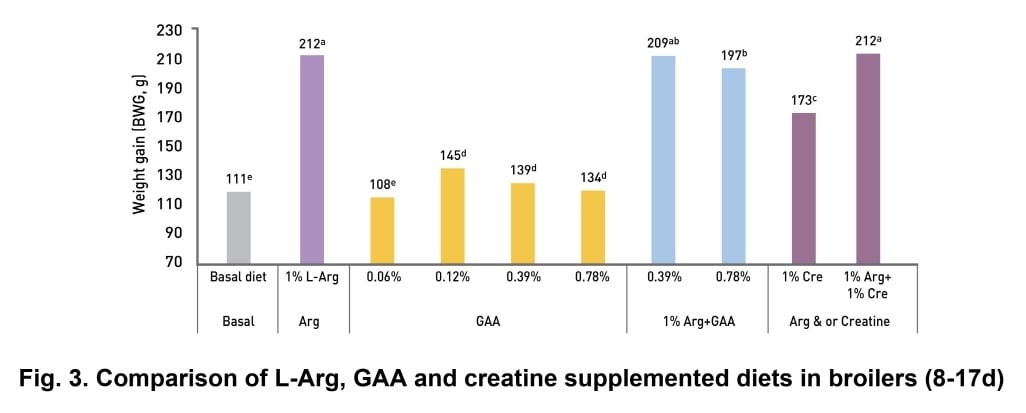
Dilger et al., (2013) showed significantly higher body weight gain (BWG) in L-Arg supplemented group as compared to diets supplemented with only GAA or creatine (p<0.05). Adding GAA and creatine on top of L-Arg does not cause any further improvement (Fig. 3).
Estimations on the arginine sparing effect of GAA
L-Arg sparing effect of GAA is not fully revealed. However, findings from literature could indicate the estimated contribution of protein bound L-Arg into GAA synthesis. In the presence of dietary GAA supplementation, we assume that no L-Arg is being used for internal GAA synthesis, which can give us idea about the Arg sparing effect of GAA.
Since the L-Arg is the essential amino acid in poultry and conditionally essential in swine, there are specific dietary requirements for the L-Arg contributed by the raw materials. De novo Synthesis of GAA is contributed by Gly, Met and Arg. Gly molecule is used as such, however, in case of Met only methyl group and in case of Arg only amidino group are incorporated. In both situations, Met and Arg can be resynthesized in their respective cycles (Brosnan et al., 2009).
Brosnan et al., (2009) calculated the contribution of the three amino acids in the creatine synthesis in growing piglets. Around 35% of dietary Met and 20% of dietary Arg contribute to the synthesis of creatine. Whereas, Wu et al., (2004) have estimated that around 17% of milk Arg may be used for creatine synthesis in piglets. Thus, by using GAA, animal needs 35% extra methionine and can save 20% of dietary Arg. Moreover, Luiking et. al., (2012) concluded that creatine synthesis consumes around 20-30% of the Args amidino groups, whether provided in the diet or synthesized within body. These findings indicate that if sufficient GAA is supplemented in the animal diet, it may spare up to 20-30% of the protein deposited Arg.
References
1. Brosnan, John T., P. Enoka, P. Wijekoon, Lori Warford-Woolgar, Nathalie L. Trottier, Margaret E. Brosnan, Janet A. Brunton, Robert Bertolo, F.P. 2009. Creatine Synthesis Is a Major Metabolic Process in Neonatal Piglets and Has Important Implications for Amino Acid Metabolism and Methyl Balance. J. Nut. 139: 12921297
2. Chen, J., M. Wang, Y. Kong, H. Ma and Zou, S. 2011. Comparison of the novel compounds creatine and pyruvateon lipid and protein
metabolism in broiler chickens. Animal. 5: 1082-1089
3. Dilger, R. N., K. Bryant-Angeloni, R. L. Payne, A. Lemme, Parsons, C.M. 2013. Dietary guanidino acetic acid is an efficacious replacement
for arginine for young chicks. Poult. Sci. 92: 171-177
4. Emami N., Golian, A., Rhoads, D. D. and Danesh Mesgaran, M. 2017. Interactive effects of temperature and dietary supplementation of arginine or guanidinoacetic acid on nutritional and physiological responses in male broiler chickens, Br. Poult. Sci. 58: 87-94
5. Fouad, A.M., W. Chen, D. Ruan, S. Wang, W.G. Xia and Zheng, C. T. 2016. Impact of heat stress on meat, egg quality, immunity and fertility in poultry and nutritional factors that overcome these effects: A review. Int. J. Poult. Sci. 15: 81-95
6. Jobgen, W. S., S. K. Fried, W. J. Fu, C. J. Meininger and Wu, G. 2006. Regulatory role for the arginine-nitric oxide pathway in metabolism of energy substrates. J. Nutr. Biochem. 17: 571-588
7. Khajali, F. and Wideman, R. F. 2010. Dietary arginine: Metabolic, environmental, immunological and physiological interrelationships.
World's Poult. Sci. J. 66: 751-766
8. Leeson, S., and J. D. Summers. 2001. Scotts Nutrition of the Chicken. Publ. Univ. Books, Guelph, Ontario Canada
9. Luiking, Yvette C., A.M. Gabriella Ten Have, R. Wolfe, and Nicolaas E. P. Deutz. 2012. Arginine de novo and nitric oxide production in disease states. Am. J. Phy.-Endo. Meta. 303: 1177-1189
10. Saki, A., Haghighat, M. and Khajali, F. 2013. Supplemental arginine administered in ovo or in the feed reduces the susceptibility of broilers to pulmonary hypertension syndrome. Br. Poult. Sci. 54: 75-580
11. M. Takeda, I. Kiyatake, H. Koide, K. Y. Jung, H. Endou. 1992. Biosynthesis of guanidinoacetic acid in isolated renal tubules. Eur J Clin Chem Clin Biochem. 30: 325-331
12. Wu G., Knabe D. A., Kim S. W. 2004. Arginine nutrition in neonatal pigs. J. Nutr. 134:S2783-90.
13. Wu, G., Bazer, F. W., Davis, T. A., Jaeger, L. A., Johnson, G.A., Kim, S. W., Knabe, D.A., Meininger, C., Spencer, T. E. and Yin, Y. 2007. Review article important roles for the arginine family of amino acids in swine nutrition and production. Live. Sci. 112: 8-22























